
(800) 429-3205
Learn More About the Investigation Into
Boston Scientific's Board Of Directors for Potential Breach of Fiduciary Duties
Boston Scientific's Board Of Directors for Potential Breach of Fiduciary Duties
Thank you! Your form has been successfully submitted.
About the Investigation
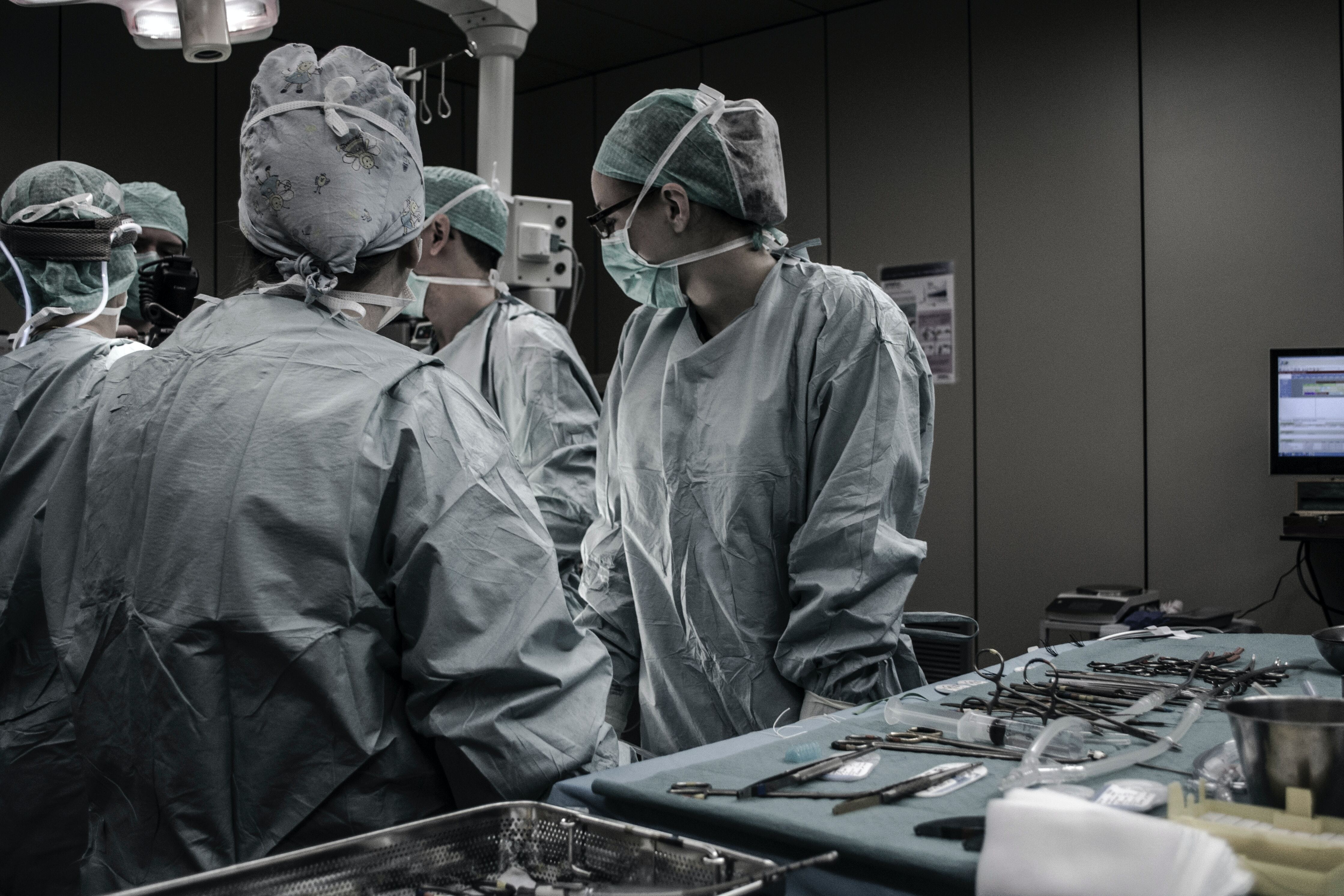
On November 17, 2020, Boston Scientific announced a global recall of all unused inventory of its LOTUS Edge, a transcatheter aortic valve replacement device used to treat patients with aortic valve stenosis. The recall was instituted on account of “complexities associated with the product delivery system.” The Company stated that “[g]iven the additional time and investment required to develop and reintroduce an enhanced delivery system, the company has chosen to retire the entire LOTUS product platform immediately.”
If you are a Boston Scientific shareholder and would like to learn more about our investigation and how it may affect you, please contact Berger Montague using the form above.
Berger Montague Counsel




Andrew Abramowitz, Esq.
25%
aabramowitz@bm.net
(215) 875-3015
bergermontague.com
Data set




Russell Paul, Esq.
25%
rpaul@bm.net
(215) 875-4601
bergermontague.com
Data set



Feature
25%
Et has minim elitr intellegat. Mea aeterno eleifend antiopam ad, nam no suscipit quaerendum.
Data set




Feature
25%
Et has minim elitr intellegat. Mea aeterno eleifend antiopam ad, nam no suscipit quaerendum.
Data set




Feature
25%
Et has minim elitr intellegat. Mea aeterno eleifend antiopam ad, nam no suscipit quaerendum.
Data set




Feature
25%
Et has minim elitr intellegat. Mea aeterno eleifend antiopam ad, nam no suscipit quaerendum.
Data set




Feature
25%
Et has minim elitr intellegat. Mea aeterno eleifend antiopam ad, nam no suscipit quaerendum.
Data set




Feature
25%
Et has minim elitr intellegat. Mea aeterno eleifend antiopam ad, nam no suscipit quaerendum.
Data set
